

Review Article - (2022) Volume 12, Issue 3
This paper is a retrospective review of 60 years of contributions by the author to chemical education. Topics covered are: chemical education research, demonstrations, laboratory instruction, teaching thermodynamics, homework, testing, Whimby pairs, problem solving, misconceptions, rote learning, and cognitive levels. A brief discussion is given of six essays entitled on the importance of: being impressive, the opening lecture, being eccentric, being passionate, rote learning, and being polite. There is also a discussion of cognitive levels relating to teaching with an example of how to present chemical reactions realistically.
When I started my career in academia in 1957 at the Illinois Institute of Technology (Chicago) I decided that I would concentrate in two areas: chemical education and research in physical chemistry, especially the thermodynamics of solutions. I have published about 80 papers in the first area and 100 papers in the latter. Since I knew that I was not competent in the area of theory I decided that I would concentrate on doing precise measurements, especially in my best known area which is the solubility of gases in liquids (The high precision measurements of the solubility of gases in water carried out by my research group was of the order of 0.02% which is about two orders of magnitude better than most measurements in the literature). My first chemical education publication was in 1960 and was on “laboratory by discussion.” I will briefly describe here some of my papers which I believe are still currently relevant. I will also describe some of the approaches I used as a chemistry professor that I have not described earlier.
Perhaps most important for my involvement with the subject of chemistry is the sense of wonder instilled in me by a high school teacher. His illustration of something at which to marvel was the reaction between metallic sodium and gaseous chlorine. Somehow, a soft reactive metal and a toxic green gas formed salt, NaCl, which is one of the most important chemicals in life (the “salt of the earth”). That very reactive metal and a noxious gas combine to make a substance absolutely essential to life. And, they do it in the spectacular fashion which he safely demonstrated. He also gave many examples of chemistry being everywhere – as in the fact that every breath we take uses the oxygen in the air and then emits carbon dioxide. In our daily lives there are endless examples of chemical reactions that we can continue to wonder about. One of my earliest papers explored the sense of wonder. This is probably what enticed most chemists to become teachers or work in the field.
At the general education level both chemistry and physics are probably the most difficult subjects for students. They require mathematical skills (algebra at the minimum), memory, and the ability to think abstractly [1]. According to Piaget’s developmental levels (and also modern considerations of constructivism) the last item is the one that is most important since much of the content of general chemistry requires the ability to think abstractly. A primary consideration here is both what percentage of freshman chemistry students have attained Piaget’s developmental level of formal operational thinking, and then how much of the material in a first year course requires such an ability. Research apparently shows that only about 10% of the U.S. adult population ever fully attains formal operational thinking skills [2]. Some studies show that 30%-40% of high school students attain such skills, and other studies show that freshman college students (depending on the admission requirements of the college, of course) are in the range of 10%-50%. Some studies have shown that the content of a general chemistry course can be over 90% abstract. This means that the prudent lecturer first needs to be cognizant of these statistics, and second needs to adapt his/her teaching methods to match students’ cognitive abilities [3,4]. Herron called attention to this in two early papers, and most recently wrote about progress in this area in 2008.
Before continuing, Renner’s relevant paper needs a few comments [5]. Essentially he points out that there are two theories of learning:
The first theory, which may be called Theory A, leads students to master the content just as a teacher gives it to them. That mastery is then usually demonstrated by performance on a test of some kind. The second theory, which may be called Theory B, leads students to develop understandings of content that are their own, not the teacher’s.
From my perspective the most important message with regard to teaching general chemistry from Piaget, Herron, Renner and constructivism is that students need to build upon their life experiences. A significant number of them will be in the concrete operational mode of development, and that means the content of the subject needs to be presented to them experientially. Power Point slides and words and symbols are all remote from experience. In this retrospective paper are presented methods that I and others have developed to connect “concretely” with students. Since I have a passion for chemical thermodynamics it is relevant to begin with the reasons why thermodynamics should NOT be part of general chemistry courses.
In 1979 this journal published two side by side articles: one by Campbell on why thermodynamics should be taught in freshman chemistry, and one by this author on why it should not [6]. My perusal of recent chemistry texts indicates that the reasons for not teaching thermodynamics to freshman are now even more important since these books contain much material on the subject [7]. One of these books uses the terminology of “internal energy” and “state functions” in a discussion of the First Law of Thermodynamics not knowing that there is no such thing as “internal energy.” Also, to properly understand the First Law you need to know something about partial differential equations and the difference between exact and inexact differentials. The First Law may be presented by the following two equations:

Where the lower case d denotes an exact differential and the upper case D denotes an inexact differential. E is the energy function (a state property), Q is the heat effect and W is the work effect. When a change of state occurs the change in the energy function depends only on the end states. When a heat effect or a work effect is determined for a given change of state their magnitudes depend on the path between the two states, or how you get from one state to the other. The marvel of the First Law, if you will, is that the sum of two inexact differentials yields an exact one. The point here is that the First Law cannot be understood or properly used with out knowledge of exact and inexact differentials, and also about the difference between state functions and effects.
One more example should suffice, and that is a consideration of the Second Law of Thermodynamics. This involve the Gibbs function (or Gibbs energy) G, the enthalpy function H, and the entropy function S. [Note that there is no such thing as a “free energy” which many texts use as a synonym for the Gibbs energy, and that the term “free energy” was discarded decades ago.] Let’s start with the definition of the entropy function:

Note three important things in this equation: (1) the entropy function is an exact differential; (2) the heat effect is an inexact differential; and (3) there is a condition of reversibility on determining changes in the heat effect. That is, changes in the entropy function can only be determined along reversible paths which must be carefully described. Without understanding these distinctions the Second Law and changes in the entropy function will be incorrect.
Changes in the Gibbs energy may be calculated from:

The Gibbs energy is a state function and is calculated from changes in two other state functions. But, note that changes in one of those functions (entropy) can only be determined along reversible paths. This means that changes in the Gibbs energy can only be determined along reversible paths. One additional comment here is that there are no entropy meters and that there are many constraints upon measuring changes in it. The entropy function is quite an abstract concept!
Does thermodynamics have a place in freshman chemistry? It has a very limited place. Thermochemistry via Hess’ Law can be dealt with readily both in lecture and lab on a concrete basis. As for the rest of thermodynamics, it is just not needed: we can work mass action problems and equilibrium problems and electrochemical problems very well without it. To say a substance dissolves because its “entropy” has increased despite an endothermic enthalpy change of solution does not add much to the everyday observation that you can indeed dissolve salt in water and sugar in coffee.
Examples of Concrete Operational Demonstrations and Activities
Laboratory by discussion: Laboratories where manuals are used for experiments have been described by students as “cookbook chemistry,” and have been used by students and teachers as crutches. The procedure described in this paper has been used for large and small classes at the freshman level and higher [1]. In effect, the teacher poses a problem to the lecture class (about 15 minutes are spent on this) in the week before the lab session concerning how to determine a particular chemical phenomenon. Via a guided discussion the class designs the experiment, and the teacher makes sure that the necessary equipment and supplies are available. The notes that the students take then become the instructions for carrying out the experiment. Thus the students are involved in designing the procedures, and they can only do the experiment if they listened to the discussion and took notes.
Student laboratory experts: In the physical chemistry and instrumentation laboratories (and other upper level ones) where there are individual stations for individual laboratory experiments the first lab meeting is devoted to discussing those experiments, and then asking students to pick the experiment for which they wish to become the lab expert. Once they choose an experiment, they immediately began research on how to do the experiment using various available references (they are also encouraged to use the library). Some of the experiments use teams of two or more students to develop the instructions for doing them. They were then printed for others to use. After this, if a student has a question about procedures or the equipment, they are told to first consult their fellow experts. This (effectively) results in a class of student teaching assistants! The teacher was the resource of last resort when questions could not be answered. So, rather than these advanced laboratories being “cookbook,” the student learn about doing research and become deeply involved.
Take home exams for upper level courses: Exams can be used to find out what the students have learned they can also be used as learning experiences. In the upper level courses I taught (physical chemistry, instrumentation, and thermodynamics) students were given one week to do take home exams. They were told that they could consult anything that was inanimate, and also the teacher if they got stumped. These consultations were by phone, Email, and in person students were given hints about how to solve particular questions on the exam. The goal was to make the exam a learning experience where everyone succeeded.
Homework: On the first day of the chemical thermodynamics course I took in graduate school the professor said something like, “You don’t learn thermodynamics what you don’t work the problems.” So, every class session had us individually putting up on the blackboard (with our explanation) a worked-out problem. Discussion was encouraged. This doing “single combat, if you will” is a wonderful learning experience.
Term papers for advanced courses: Most graduate students find chemical thermodynamics to be one of the most difficult courses they take due the rigor of the subject, abstract concepts, and not having any idea of its practicality. On this last point note that the practical subject of chemical engineering is in effect applied thermodynamics (think about manufacturing plastics or petroleum products or of engine efficiency). So, the students were required to research and write a term paper on the application of thermodynamics to any subject in which they were interested. The last class session was devoted to each student doing a presentation based on their term paper, and the variety of subjects was always amazing! Again involving students in their own learning works.
General chemistry multiple choice exams as a learning experience: At the first regular class (the “real” first class was a chemistry demonstration show) students were given the four multiple choice exams for that quarter (or term) along with the answers printed upside down on the last page. Two exams were given during the term, and two one hour exams were given at final exam time. The students were told that the “real” exams would be identical except for: (1) the questions and answers would be scrambled; (2) numerical questions would have different values and would be for different substances; and (3) different substances would be used as in a different compound for calculating a molecular mass or balancing an equation. Rather than making an exam a guessing game, students were told at the beginning of the course exactly what the teacher wanted them to learn.
Chemistry demonstrations: The subject of chemistry is all about the world around us and how it changes. We ourselves are chemical factories taking in oxygen breathing out carbon dioxide, and chemical reactions in our bodies produce the heat that keeps us warm and the energies that tighten muscles and the digestion system that processes the food we eat. We can wonder (2) how these things come about and how we can entrance our students to explore chemical reactions and phenomena. Assuming that constructivism is correct, and then it is imperative that students experience these phenomena not by images on a screen, but in real life circumstances. This is where chemistry demonstrations and laboratory experimentation enter. Observing demonstrations in real time is great, and demonstrations where students act out various phenomena are even better [8]. In this paper various phenomena are acted out at the front of the lecture hall using student volunteers. The phenomena are: chemical kinetics, chromatography, balancing equations, gas laws, kinetic molecular theory of matter, Henry’s law of gas solubility, electronic energy levels of atoms, translational and vibrational and rotational energies of molecules, and organic chemistry (there are photographs of these activities) [9]. Dynamic equilibrium is illustrated in, and giant models for atoms and molecules and spdf sandwich boards for electronic energy levels are shown in [10].
In doing demonstrations it is useful to occasionally chuckle which sets the stage for something interesting coming up. Also, we found that it is important to talk continuously when doing demonstrations-pauses lose attention.
For many years it was the practice of the author to do a full period chemistry demonstration at the first class session. The idea was via a kind of Pavlovian or an operant conditioning experience to establish that this lecture hall as an interesting place to be, and the subject of chemistry is fascinating and enjoyable. Also, it was not unusual to just do an interesting demonstration from time to time even if it were not connected to the topic of the day. That is, it is useful for the lecturer to be unpredictable. Along this line telling random stories (relevant or not) is helpful. Student feedback at later times showed that the students tended to forget chemistry, but remembered the stories!
The author and his colleague John J. Forman did free chemistry outreach 90 minute demonstration shows for middle and high school students for over 40 years. (To see the 1908 professionally produced shows go to: https://videoplayer.telvue.com/player/UkHhwMt7D6u3LBSmJKr3kgnqPEIbxak7/ media/226985?fullscreen=false&showtabssearch=true&autostart=false. Individual demonstrations can be found on YouTube under the author’s name.)
Three minute end of lecture questions: Rather than wait to find out what students have learned via exams you can get instant feedback at the end of a lecture via three minute end of lecture questions. Students answer the following questions and turn in their answers:
1. What are your main take aways from today’s lecture?
2. What didn’t you understand in today’s lecture that needs further explanation?
3. Do you have any other comments on today’s lecture?
These questions will guide you on the content of your next lecture!
Whimby Pairs: When the lecturer poses and then works out problems in class the students experience only the lecturer’s way of doing this [11]. Pestel describes in the following an in class approach that actively gets the students to think through how they would solve that problem via Whimby Pairs.
Students learn more efficiently when they are exposed to many strategies and when forced to explain what they are doing. Have the class divide into A and B adjacent students. Pose a problem like balancing an equation, calculating a pH, or a gas law [12]. Student A works the problem explaining all the time to B what s/he is doing. B listens and can ask for clarification an answer. They change roles for the next problem, and can also work with other students at other times.
Importance of Different Series
There are six papers in the “importance of” series and each is briefly described.
On the importance of being impressive: The Opening Lecture The first lecture sets expectation levels for the rest of the course. The students assess you and you assess them [12]. When the course begins with the syllabus and information about exams and requirements, etc., then you are in effect telling them to anticipate being in a demanding time in a subject which they already expect is going to be hard. If you start out (as described above) with an interesting and fun experience like a full class chemistry demonstration show, you have changed the whole atmosphere in that room to one of anticipation, i.e., this may be an interesting course!
On the importance of being eccentric: This paper begins with: “A graduate student from England once commented to me ‘This department has no right to have only one bona fide eccentric [13]. You need more.’ He felt, and rightly so, that a department containing about twenty faculty members needed at least one half dozen eccentrics. We were short changing our students.” A bit later it continues, “If you do not currently possess eccentricities, then cultivate them if you wish to be an effective lecturer.” The paper makes the point that not only do chemistry lecturers have the opportunity to be eccentric; they in effect almost have the obligation to do so!
On the importance of being passionate: If you are not overtly and openly passionate and interested in your subject, how can students be interested in it? Enthusiasm is catching, as is the sense of wonder and amazement about chemical phenomena in the world around us [14]. If you are not having fun and enjoying yourself in that lecture hall, do not be surprised if your students get bored. The author has written many skits for general chemistry that can be used for comic relief in class. They are chemically accurate, involve two student roles and that of the teacher, and take perhaps ten minutes to perform [16]. (The complete up to date set is available on request from the author as a PDF file.)
On the importance of rote learning: Does a student need to “understand” everything taught in general chemistry or are there some things that they can learn by rote (memorize) that are useful and practical? They drive a car and do not know that the engine in their car is an example of the Otto cycle, and they do not understand or know anything about the combustion reactions in the cylinders. They can do basic mathematical calculations without understanding mathematical theory. And, they use all sorts of devices daily (like smart phones and electronic calculators) without any knowledge of what is going on in the circuit boards inside them. So, there are many parts of a general chemistry course where students memorize definitions and equations and how to do simple things like calculating a molecular mass or the pH of a solution or solving a gas law problem. There are many places for rote learning in general chemistry just use them.
On the importance of being polite: Being polite means a cheery greeting upon entering class and showing how delighted you are to there. (When you act as if you are happy to be there this becomes a self-fulfilling action.) It means getting to class a bit early and chatting with a few students before class starts. It means being polite throughout the session, and thanking students for asking questions or making comments [17]. You can even tell these students that they are secretly admired by the rest of the class, because they are willing to be brave by asking simple or even “dumb” questions. Questions are repeated for the entire class.
Students are told at the beginning of the term that if I hear or see anyone talking, I will stop talking since they are being impolite to me and the class, i.e., only one person talks at a time in that room [18]. (I once stopped talking for over 15 minutes.) It is important to end the class with something like, “You know, I really enjoyed being with you today. Thanks for coming, and thanks for your attentiveness and participation.”
On the importance of ideality: The thermodynamic concept of ideality is discussed in detail with examples and illustrations, along with the concepts of reversible and irreversible processes, reversible heat and work effects, ideal and real gases, and ideal and real solutions [18].
Presenting chemical reactions realistically: The hallmark in teaching chemistry is the use of chemical equations. On the left side of an equals sign (or an arrow or a double arrow) are the reactants and on the right side are the products. There is a conceptual difficulty about this which will be illustrated via the reaction between hydrogen and oxygen to produce water:
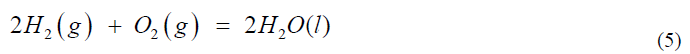
This is conceptually better represented by:

where the double arrow indicates that the reactants are not “equal” to the product, but that there is a reaction that can go in both directions. The difficulty here is that you see the reactants and the products at the same time but do not understand that when there is a dynamic equilibrium between them that you have mostly water and an incredibly small amount of hydrogen and water [20]. A more accurate presentation is indicated by changing the font sizes:
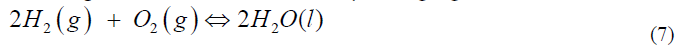
After the presentation of the concept of equilibrium constants, the magnitude of the equilibrium constant for this reaction will properly show the relationship between all of the concentrations. Another way to show that reactants are used up and products are produced is by showing the following two equations sequentially:

Another way of doing this which may be more satisfactory is using an overhead projector and small plastic letters and rearranging the reactant letters to form the products (or an animation in Power Point) is:

That is, there is no loss in matter only a rearrangement of the elements from one set of compounds to another set of compounds (Ref. 9 shows how this can be done using students holding placards indicating which element they represent). Some may consider the distinctions made in this section to be trivial, yet for any observer whose developmental level is concrete operational this makes great sense.
A demonstration using the Hoffman electrolysis apparatus (or a 9 V battery and two inverted test tubes filled with water in a large beaker and appropriate wires) to generate H2 and O2 gases helps. Then showing their characteristics by inserting a lighted splint in the upside down H2- filled test tube and the open side up O2-filled test tube is convincing. Exploding a small balloon filled with both H2 and O2 shows that the two gases can exist without reacting until an “energy of activation” via a flame starts the reaction.
This retrospective paper contains reminders of a number of approaches to teaching chemistry, and a few that have not been published before. Since retiring in 1995, and a subsequent stopping of a subscription to this journal, there have undoubtedly been many useful and interesting papers on the teaching of chemistry the author apologizes for not referring to them. Readers may be interested in a recent paper entitled “Comments on the Teaching of Chemistry, Doing Chemistry Demonstrations, and a Passion for Chemical Thermodynamics.”
The author acknowledges the collaboration, what he has learned from many colleagues, and their encouragement: David Karl, John J. Fortman, Joe Bitzko, Andrea Burns, Al Delfiner, David Dolson, Kirby Underwood, Henry Bent, Norman C. Craig, Scott E. Wood, Emmerich Wilhelm, Trevor M. Letcher, Pirketta Scharlin, Arthur Williamson, and many high school and undergraduate and graduate students. Howard R. DuFour, James D. Arehart and Michael R. Hall constructed the demonstration devices featured in many papers. The author are grateful to the journal editor and the anonymous reviewers for their helpful comments and suggestions.
The authors declared no potential conflicts of interest for the research, authorship, and/or publication of this article.
Received: 01-Jun-2022, Manuscript No. jesr-22-69598; Accepted: 22-Jun-2022, Pre QC No. jesr-22-69598; Editor assigned: 03-Jun-2022, Pre QC No. jesr-22-69598; Reviewed: 17-Jun-2022, QC No. jesr-22-69598; Revised: 22-Jun-2022, Manuscript No. jesr-22-69598; Published: 29-Jun-2022, DOI: 10.22521/JESR.2022.12.3.11
Copyright: © This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.